Cell/Module/Pack manufacturing
Safety & Efficiency
Proper battery thermal management ensures longer lifespan by keeping the cells within a limited temperature range during storage, operation and charging. Understanding how much heat can be dissipated by the cells requires understanding of the basic heat transfer properties of the cell design.
Electrolytes are characterized by high conductivity, good electro-chemical stability and the ability to perform at low temperatures. However, the thermal stability of many electrolyte solutions is restricted even at moderate temperatures. Due to overcharging, batteries can overheat to the point that they catch fire. Besides various metals (e.g., Co, Al, Mg, etc.), the cathode material of Li-ion batteries contains nickel. There is a positive correlation between the nickel content and the battery capacity. However, nickel reduces the stability as it reacts easily to the external environment. The nickel content can lead to deterioration in stability and must be improved to ensure safety.
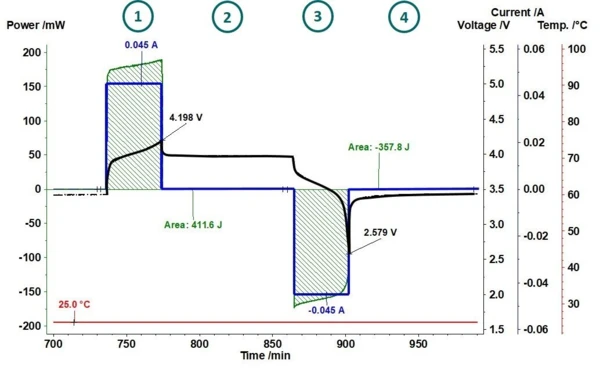
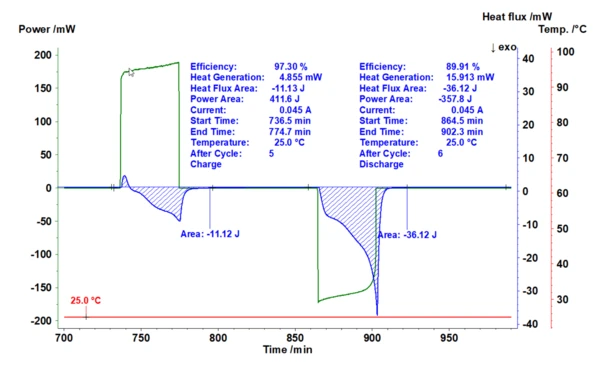
Specifically, understanding of the heat generation during charging/discharging cycles is crucial for improving the cell efficiency, performance and lifetime of batteries. Measuring the heat signature of coin cells during cycling provides insight into the underlying processes and provides a quantitative way of comparing changes in chemistry above and beyond current and voltage measurements. The amount of heat released or absorbed during all these physicochemical changes and the rate of energy change within the coin cell provide additional pieces of the puzzle and can accelerate the development process.